AbCellera
An engine for antibody drug discovery and development
DCVC Bio has led a $10 million Series A in AbCellera, the company using the immune system as a search space for therapeutic antibody discovery. Their team has built a platform that generates antibody drug candidates with disruptive speed, diversity, and quality. These improvements translate into accelerated drug development for their customers. At DCVC Bio, we support companies using new technologies to drive improvements in the life sciences industry. We look for particular qualities in the companies we support, and AbCellera exemplifies those qualities with:
a) An incredible team — the scientists running the company are the same ones who invented the techniques they are using and improving,
b) Adept application of automation and data-driven optimization, and
c) Dedication to connecting big data and disruptive technology to drive step change improvements in the life sciences industry.
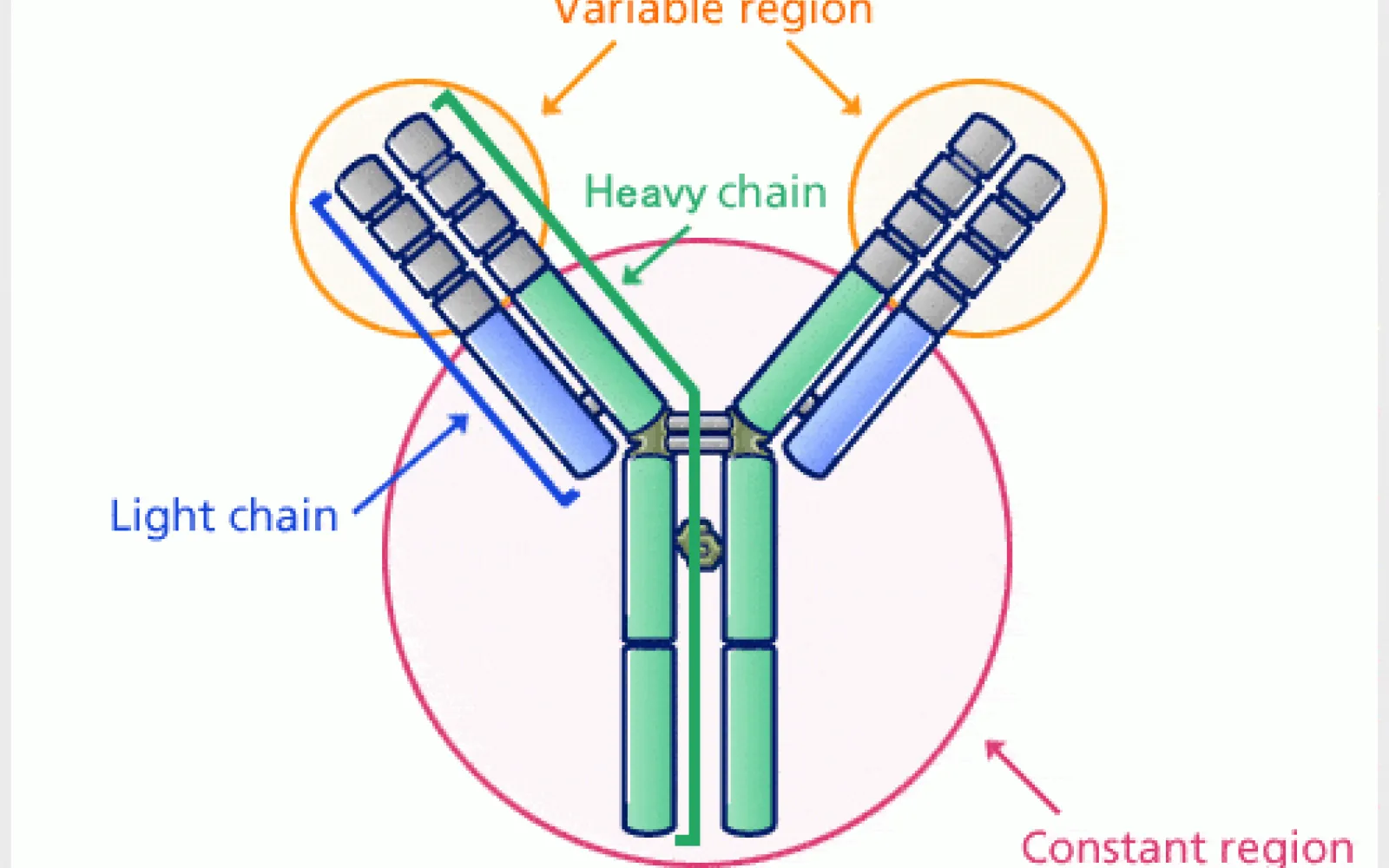
Antibodies — the molecules AbCellera’s technology is built around — are Y‑shaped defensive proteins made by the immune system. The tips of the Y’s branches bind to foreign molecules like bacteria and viruses (antigens) and mark them for destruction. These tips are variable regions — they bind to different parts of antigens and can impact them in different ways. One antibody may incapacitate a virus by binding to the site it uses to infect cells, while another antibody could mark a cancer cell, signaling the immune system to destroy it.
Antibodies come from a class of white blood cells called B cells, which undergo extreme gene shuffling to make antibodies with unique variable regions and binding properties. The human immune system can make up to one hundred trillion different antibodies, of which each of us makes about one billion at any given time. This high variety helps the immune system protect us from antigens in many different ways, but developing therapeutic antibodies requires isolating the single best-performing antibody against a disease. This means sifting through millions of B cells — about 500 million in a liter of human blood — to find the best candidate.
AbCellera has built a platform that uses microfabrication, computer vision and machine learning to screen millions of B cells at a time, searching the immune system at a depth orders of magnitude beyond what others have done before. This deep search generates more, higher-quality antibody candidates against diseases than traditional methods. It also discovers molecules against drug targets — cell membrane proteins that interact with molecules outside the cell — that have been impossible to find before. AbCellera’s technology is the next generation of antibody discovery platforms, outperforming traditional hybridoma and phage display methods in output, speed, and quality.
In the hybridoma method, scientists use rodents’ immune systems to make antibodies. They inject a mouse with the antigen they need antibodies for, which triggers the mouse’s immune response, then take a sample of the mouse’s B cells. These cells are then fused with cancerous ones to create immortal hybrids called hybridomas. The scientists grow the hybridomas in a mouse or in a culture dish, which have to support thousands to millions of B cells to produce enough antibodies for testing. Once the scientists have screened the antibodies, they send the best candidates on to further development and (hopefully) clinical trials.
Immunization, fusion, isolation and screening can take months or years. Once scientists have found a promising antibody, they isolate and clone its genes. They then prepare the antibody for clinical use, converting its rodent DNA sequences into human sequences (humanization). (Transgenic rodents with human antibody genes can produce human antibodies directly.) Still, even when well-optimized, only about 1% of cancer – B cell fusions successfully make hybridomas. As a result, many potential antibody candidates — including the ones with the best properties — are lost upfront.
As an alternative to hybridoma, scientists use microbial systems with bacteriophages (bacterial viruses) and yeast that express (display) antibodies or antibody fragments on their surface. These display systems, invented over 20 years ago, create large libraries of antibodies that can be screened by panning (washing an antibody library over a surface-bound antigen to see what sticks) or by flow-activated cell sorting (exposing an antibody-expressing cell to an antigen and keeping the cells the antigens stick to).
Compared to hybridomas, one advantage of display platforms is their ability to generate antibodies against epitopes (parts of an antigen) that are highly conserved between humans and mice; display platforms are not subject to immune tolerance, the process by which antibody responses to self-antigens (those shared between the target and the host) are suppressed.
However, display systems have disadvantages. First, they lack somatic hypermutation, the molecular process that selects and optimizes high-performing B cells in natural immune responses. As a result, antibodies from display libraries are typically low-affinity and require lengthy improvements using protein engineering. Second, antibodies obtained from display libraries have not been subject to the checks and balances of the immune system, which eliminate antibodies that are sticky (non-specific) or that don’t express well. Because of this, many antibodies derived from display libraries either clump together or are difficult to express, making them poor candidates for development. This poor developability has been a shortcoming of display platforms for decades.
Microbes engineered to express human antibody genes can make antibodies synthetically, but antibodies from natural immune responses have been far more successful — they make up about 90% of approved drugs. This is because immune responses have evolved over 150 million years to make antibodies with high specificity (don’t bind to other things), high affinity (bind tightly), and that express well (are easier to develop). Microbes in test tubes still lag far behind Mother Nature’s kitchen.
Traditional antibody discovery methods have built a $108 billion therapeutics market, but the future of that market lies with new methods. It is no longer good enough for biotech companies to find a small number of passable antibody leads. Being competitive means developing many diverse, potent, developable molecules as quickly as possible. As the industry matures, the easily accessible drug targets have been picked over, and companies are looking to more difficult target classes (small peptides, ion channels, GPCR proteins) that represent untapped areas for therapeutic antibody development.
AbCellera uses a high-capacity screening system to rapidly screen millions of antibody-producing cells from any animal, including humans. The cells are loaded into a microfluidic device — a miniaturized lab-on-a-chip the size of a credit card — with thousands of connected chambers that cells, antigens, and chemical compounds can move through in a programmable way. Each chamber has a volume of approximately 1 nanoliter, which is about 100,000 times smaller than the chambers conventionally used. Isolating individual cells in small-volume chambers allows for the sensitive measurement of the antibodies they produce. This system can interrogate each antibody-producing cell simultaneously with antigens, antigen fragments, or undesired fragments under various conditions, with readouts based on high-content microscopy and machine-learning algorithms. Advanced data visualization deconvolutes the interactions to identify the antibody-producing cells of choice. This approach creates an unprecedented “deep search” of the immune system. By going deep, AbCellera discovers antibodies that are completely invisible to hybridoma methods: potent neutralizing antibodies against difficult targets like GPCRs and ion channels; antibodies with specificity present in less than 1 in 100,000 B cells; and (overcoming tolerance) hundreds of antibodies against antigens that are 100% identical with the host. AbCellera analyzes the entire antibody repertoire to identify hundreds of drug candidates, and combines these sequences with data from automated assays testing antibody-epitope recognition, affinity, developability, and potency. This creates a powerful engine for the discovery and selection of high-quality antibody candidates for drugs.
So far, AbCellera has been discovering therapeutic antibodies for infectious diseases (e.g. Ebola, drug-resistant bacteria and influenza), fibrosis, muscular dystrophy and a variety of cancers and neurodegenerative diseases. They recently partnered with the DARPA on their Pandemic Prevention Platform to develop rapid countermeasures to viral pandemics. It’s been rewarding to see AbCellera working on treatments for so many critical diseases, and the company continues to form new partnerships with leaders in industry and academia.
AbCellera’s is currently scaling up operations at a new office in Vancouver, and we’re proud to support them as they do. We invested in AbCellera because of their team’s technical expertise, their adept application of data and automation technology, and the fundamental changes their inventions make to the antibody discovery process. We are especially glad to back founder and CEO Carl Hansen (who studied under Chan-Zuckerberg BioHub Director Steve Quake and biotech legend Leroy Hood). AbCellera will dramatically accelerate drug discovery by merging artificial intelligence, microfluidic technology, and immunology, backing this technology with just the right mix of passion, innovation, and grit. As the biotherapeutics industry continues to expand, we’re excited to see how AbCellera will help companies find tomorrow’s cures.