Grove Biopharma
Putting protein-like drugs inside cells to fight cancer and neurodegeneration
Protein-to-protein interactions inside cells make up the balanced kinetic dance that keeps cells running. Mutations to any number of these key proteins can lead to disorder and disease — especially when it comes to cancers. These protein-protein interactions have always posed a challenge for drug makers searching for ways to disrupt these interactions. Scientists have successfully developed many protein drugs — such as antibodies — that can alter extracellular protein-protein signaling. Unfortunately, we have always had a hard time making drugs for the inner cell environment that are a) either small enough to penetrate the cell membrane or can be transported across the cell membrane, but b) large/ charged/ specific enough to bind to target proteins inside the cell, and c) nimble enough to evade the proteolytic enzymes that are always trying to break down foreign molecules inside cells.
Traditionally, drug discovery has focused on small-molecule therapeutics, typically with a molecular weight of less than 500 daltons. However, with the increasing influence of AI in drug discovery, there is increased interest recently in so-called ‘beyond rule-of-five’ compounds, such as macrocycles. While small molecules typically contain rings of five to seven atoms, which are relatively inflexible, macrocycles usually contain 12 or more atoms in a ring, although they can be significantly larger. The larger rings within these molecules create a higher degree of flexibility, enabling them to adopt a large range of conformations. Their larger surface area also allows greater interaction with the target. Thus they bind to their targets with higher affinity and selectivity than many non-macrocyclic drugs.
Typically, small-molecule drugs target active sites buried inside proteins. However, some active sites are large, flat sites or shallow grooves on the surface of the protein. These sites are commonly involved in protein-protein interactions, some of which are key to disease development.
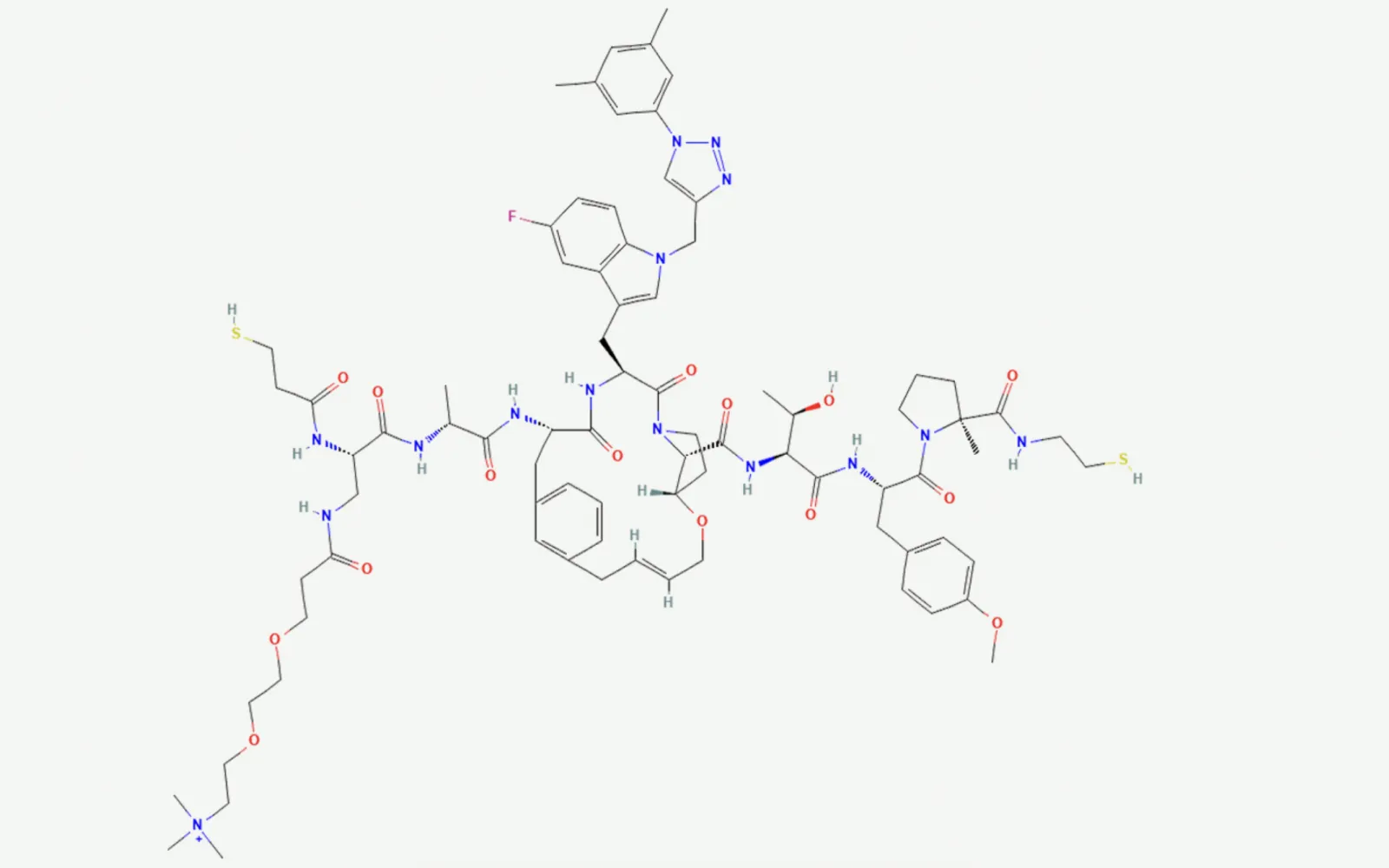
Over the last 15 years, one promising answer has emerged: synthetic macrocyclic peptides. Macrocycles — ring-shaped molecules — offer a unique “Goldilocks” modality. They’re larger and more versatile than typical small molecules, yet smaller and more drug-like than biologics. Their size and conformational flexibility enable them to intervene in protein – protein interactions previously considered “undruggable.” Examples in development include HCV treatments like grazoprevir and cardiovascular agents such as the oral PCSK9 inhibitor MK‑0616, which is entering Phase III trials (see image).
Macrocycles are most notoriously naturally produced by bacteria, fungi, and marine organisms, which have evolved these molecules as an armament for the inter-microbial warfare that occurs in soils and other environments. Humans have been trying to tweak these natural products to turn them into drugs for decades. There are now more than a dozen FDA-approved macrocyclic peptide drugs, for everything from cancer to kidney disease to candidiasis — they make up about 4 percent of drugs. However, these molecules are difficult to alter to direct them to new targets, as adjusting one feature typically costs the molecule its ability to enter cells, avoid premature degradation, bind the desired target specifically, or be manufacturable.
This multiparameter optimization problem has been resistant to AI improvements (like AlphaFold and derivatives) for a variety of reasons — mostly that the empirical data sets for feature recognition continue to be expensive and hard to produce. Macrocycles are not engineerable today, yet. At DCVC Bio, we’ve consistently sought out platforms that can rationally design and efficiently assemble protein-scale therapeutics — capable of modulating historically intractable intracellular targets through novel scaffolds, improved cell permeability, and tunable pharmacokinetics. Our optimism has always been rooted in the evolving convergence of synthetic biology, structural informatics, and molecular engineering.
When we crossed paths with the team at Grove Biopharma, we saw a solution. Grove is led by scientific founder Dr. Nathan Gianneschi of Northwestern University, and CEO Dr. Geoffrey Duyk, whose leadership pedigree spans Exelixis and Millennium Pharmaceuticals. Back in April, we led Grove’s $30 million Series A, and since then, we’ve watched with fascination as they’ve repurposed non-drug-like peptides into viable drug candidates using the elegant chemistry of ring-opening metathesis polymerization (ROMP). The group is crafting bottle-brush-like polymers that are able to specifically bind unstructured targets in oncology, inflammation, and beyond.
PLPs or Bionic Biologics are fully synthetic protein mimetics purpose built to target classically intractable intracellular therapeutic drug targets (e.g. targeting intrinsically disordered regions of proteins or protein-protein interactions). They represent protein-scale molecules for protein-scaled solutions. Proteins can be considered precision polymers of amino acids (polyamides), but in contrast PLPs or Bionic Biologics are precision bihybrid branched polymers of peptides (brush bottle polymers) built using living graft through polymerization of peptides monomers using ROMP. These dynamic macro monomers have a number of emergent properties that overcome the limitations of peptides and macrocyclic peptide chemistry including but not limited to cell permeability, pH/protease resistance, avidity driven enhancement of potency, microenvironment-induced predicted secondary/tertiary structure of peptides without requirement for covalent modification, and adaptive binding to targets, including all defined classes of protein-protein interaction surfaces. In addition, PLP chemistry avoids the synthetic and manufacturing complexity of macrocyclic peptides and related chemistry. In contrast to alternative chemistries, PLPs are modular, allowing the uncoupling of optimization of potency from pharmacology, and are readily configured into multifunctional molecules enabling proximity-induced chemistries, subcellular localization, and delivery of payloads. PLPs (20−40 kilodaltons versus 0.5−2 kilodaltons for macrocyclics) represent protein-scale molecules for protein-scaled solutions.
The resulting molecules are multitalented. When the peptide bristles are folded up into a ball around the backbone, they’re highly polar and water-soluble, meaning they can travel well in the aqueous environments outside cells. But at the cell wall, the PLPs can unfold, exposing the low-polarity backbone, which allows the molecules to dissolve through the phospholipid bilayers that makes up all cell membranes. Once inside, the molecules fold up into a ball again for protection from proteolytic degradation. PLPs are programmable, too, in the sense that the amino acid sequences in each bristle can be tuned to bind to specific proteins inside cells and block processes that can lead to disease.
One of the first clinical targets Grove is going after is prostate cancer. Cancer cells in the prostate need testosterone to grow, which is why hormone therapy to block androgen production is a common treatment choice. But even in the absence of androgens, the signaling pathway sparking cell growth can be reactivated if tumor cells happen to acquire a mutant androgen receptor called AR-V7.
Grove has built, and is currently testing, a PLP that targets and degrades both the wild-type androgen receptor and AR-V7. The advantage of starting with a PLP that degrades AR-V7 is that androgen receptor antagonists are a well understood and validated drug category with a clear unmet need — providing a developed test for Grove’s technology.
Beyond that, though, Grove is designing PLPs that can disrupt SHOC2, a crucial scaffolding protein involved in the RAS/RAF/MAPK signaling pathway that controls cell division and differentiation, including in cancer. Several other targets round out their aspirations to create the first PLP drug modality platform.
PLPs will provide a totally new modality for treating cancer and other common health problems — one that will compete with and (thanks to PLP’s manufacturability) likely surpass macrocyclic peptides to become the antibody for inside cells. We think the Grove team is brilliantly qualified to pursue this vision, and we’re excited to follow their progress toward clinical trials.