Umoja
Pioneering the future of immunotherapy
The DCVC Deep Tech Opportunities Report (which debuted this year) summarizes our thinking about the deep tech investment areas we consider the most exciting, important, and consequential. It’s also a guide to the inspiring work innovators inside and outside the firm’s portfolio are doing to extend human capabilities, save the environment, and make everyone’s lives longer, healthier, and easier.
Three of this report’s opportunities bore on health and life sciences. This is one of them.
The messenger RNA vaccines developed during the coronavirus pandemic showed that our own cells can be repurposed as microbial protein factories, teaching the body how to defend against viral invaders. But mRNA vaccines are only the beginning. Commercial oncology treatments that use genetic modification to convert our immune cells into cancer fighters are already available, and now biotech innovators are pursuing similar techniques that could teach our cells how to fight cancer or even permanently correct the genetic changes that cause many common and rare conditions. “We’re investing in treatments that are one-shot, treatments where you produce that drug for life,” says DCVC Bio managing partner Kiersten Stead, who earned a PhD in molecular biology and genetics. “They’re using you as the manufacturing vehicle.”
Some of the original leads in cell engineering came from the field of cancer immunotherapy. More than two decades ago, researchers began designing artificial versions of T‑cell receptors: the proteins on the surfaces of some white blood cells that recognize antigens and initiate an immune response. They hoped T cells reprogrammed to express these chimeric antigen receptors (CARs) would recognize, target, and destroy cancer cells. The idea worked. There are now six FDA-approved CAR‑T cell therapies for blood cancers such as lymphoblastic leukemia, B‑cell lymphoma, and myeloma. They are remarkably effective, helping many patients achieve lasting remission.
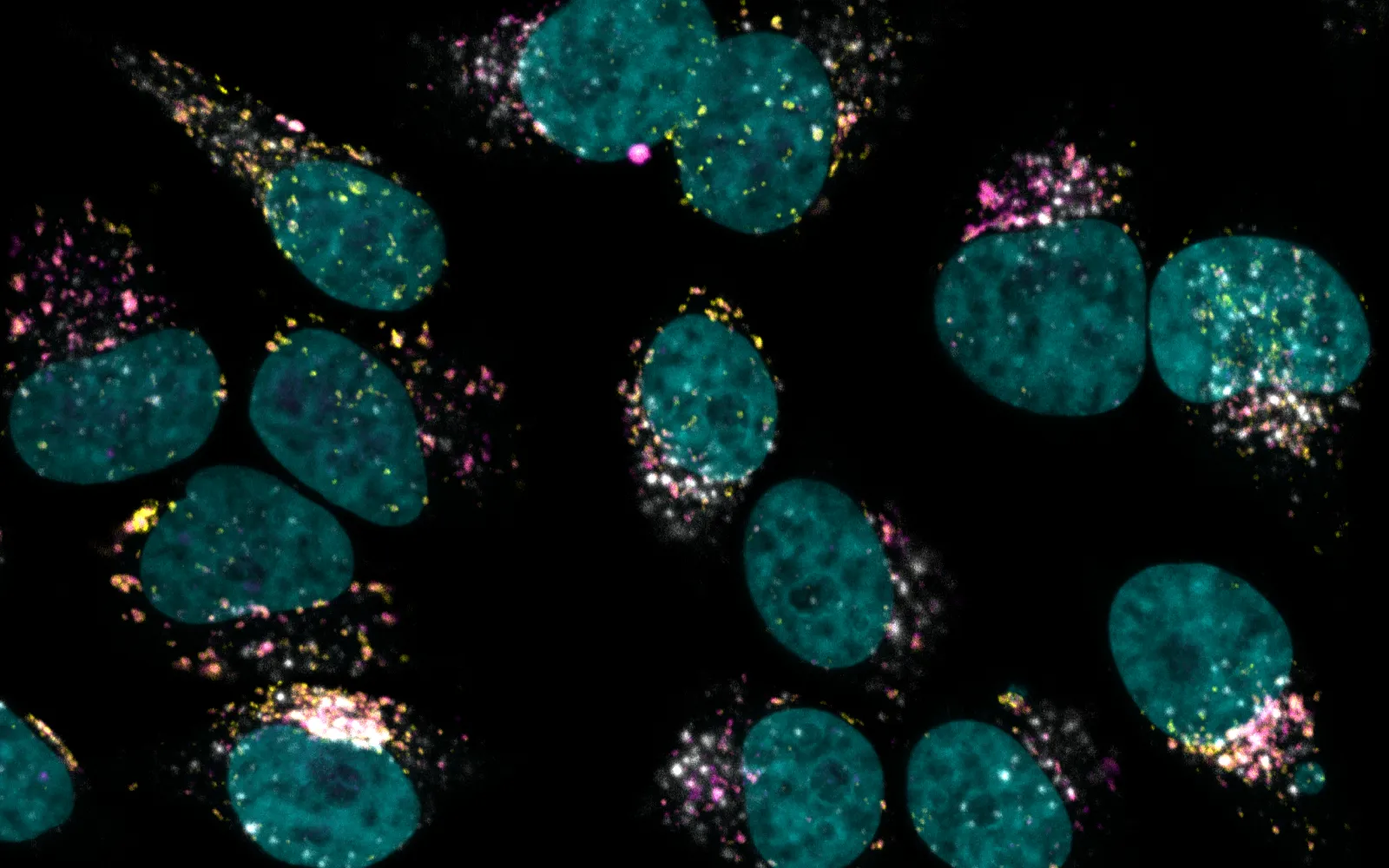
But CAR‑T therapy is slow, expensive, and fraught with side effects; for one thing, patients whose white blood cells are removed and transfected in the lab with the required DNA must also undergo chemotherapy to kill most of their remaining white blood cells, leaving them vulnerable to infection in the period before retransfusion. It would be faster, safer, and cheaper to teach a cancer patient’s T cells how to make tumor-targeting receptors without ever removing the cells from the body. And that’s exactly the approach taken by Umoja Biopharma, one of DCVC Bio’s portfolio companies.
Umoja encodes the instructions for a receptor targeting cancer cells into RNA. It puts that RNA inside a protein capsid shell and an outer lipid envelope. Once the vector enters a white blood cell, the RNA is reverse-transcribed into DNA and integrated into the cell’s genome, where it directs expression of the chimeric receptor and equips the cell to attack tumors. All this happens without extraction, transfusion, or chemotherapy. The process can even be turned on and off at will: Umoja tailors the new RNA so that the receptor-making genes are activated only when a safe, approved small-molecule drug related to rapamycin is present.
The company’s scientists believe this approach will lower the overall risks and costs of cancer treatment, and provide a built-in hedge against recurrence. “Once those cells are created, they’re engineered inside you forever,” Stead explains. “This old paradigm of going into remission, coming out of remission, and having to increase the aggression of your cancer treatments is going to be something that we can get around, because we will just re-induce that cell system through the action of the small molecule.”
DCVC-backed Kanvas Biosciences is exploring ways to control a different population of cells: the gut microbiome. Kanvas is using RNA mapping of both microbial and host cells to figure out not just which microbes are present in our bodies, but exactly where they are and what effects they’re having. In one experiment, for example, Kanvas was able to use its multiplexed fluorophore tagging system for RNA— combined with spectral imaging and a form of machine learning — to show that a new cocktail of beneficial bacteria is able to protect against an overpopulation of Gardnerella vaginalis, a type of bacteria that can cause vaginosis.
“The microbiome is not only critical to human health, but should be considered a separate, distinct organ system within the human body,” Cheng says. “And because of our ability to look not only at what’s happening in the microbiome, but also in real time see what’s happening in the host, we believe we have a unique opportunity to design the next generation of microbiome therapeutics — to see how they engraft, look for safety, and look for efficacy, all in the same assay.”
The more researchers learn about how to alter gene expression in cells, how to get genetic payloads into the body, and how to work with (rather than against) the human immune system and microbiome, the sooner they will be able to intervene against a range of stubborn health problems. “This is a trend that is in flight or at the beginning of being in flight, and I don’t think there’s a lot of visibility into it” among investors, says Stead. “But it’s something we think is going to be transformational.”
Read and/or download the Deep Tech Opportunities Report here.