*This post has been updated as of 4/19/20 to reflect new guidance from the FDA.
Our world has been turned upside down by COVID-19 in just a few short months. This crisis impacts every aspect of daily life, and our hearts go out to the individuals and families affected by this pandemic.
America and the world now face the simultaneous challenges of developing treatments and vaccines, creating and distributing fast testing capability, and delivering diagnoses and healthcare outside of increasingly over-burdened hospitals.
The DCVC Deep Tech portfolio has sprung into action across all of these fronts, delivering concrete help that can bend the curve on these difficult challenges. We invested in these brilliant teams because of their dedication to solving society-scale problems with a unique combination of speed, efficacy, and cost-effectiveness. They are by definition well-equipped and deeply committed to respond to and help mitigate a crisis like the SARS-CoV2 coronavirus pandemic.
Some examples of these concrete actions include:
- DCVC Bio company AbCellera, which announced an agreement with pharmaceutical giant Eli Lilly to co-develop antibody products for the treatment and prevention of COVID-19. AbCellera identified 500 viable candidates within 11 days of receiving a virus sample, and the two companies will ideally be testing these candidates in clinic within 100 days.
- Curative which provides an FDA-authorized oral fluid test for COVID-19. This highly accurate oral fluid swab test has an average one day turnaround (with samples mailed to a lab which can process samples in as little as 3 hours at full levels of automation). Curative leverages an orthogonal supply chain (different materials, different swabs) and requires zero personal protective equipment to administer. Currently used at drive-through and other test sites in Los Angeles, Curative has tested more than 57,000 people and processes approximately 5,000 tests per day. Its platform can be scaled (via multiple labs) to enable nationwide testing that also tracks community spread and dangerous mutations.
- Carbon Health, which uses ML and advanced software to deliver accessible, higher quality and more empathetic urgent care and primary care across California. Its comprehensive COVID-19 response includes online symptom assessment tools and comprehensive telemedicine services – the company has completed more than 2,000 assessments and 300 tests in the clinic to date. It has also capped out of pocket medical costs for people who are uninsured and is building a database of testing centers across California to connect patients with healthcare facilities closest to them.
- Atomwise, which has three active programs to discover broad-spectrum antivirals for coronaviruses that could treat SARS-CoV‑2, SARS-CoV‑1, MERS-CoV, with the goal of future proofing against related outbreaks in the future. Using AI to speed the discovery of novel antivirals won’t impact the current pandemic because of the long regulatory path to bring new drugs to market, but broad-spectrum antivirals that can not only treat COVID-19, but also ‑20, ‑21 and ‑22 will ensure we’re prepared for the future.
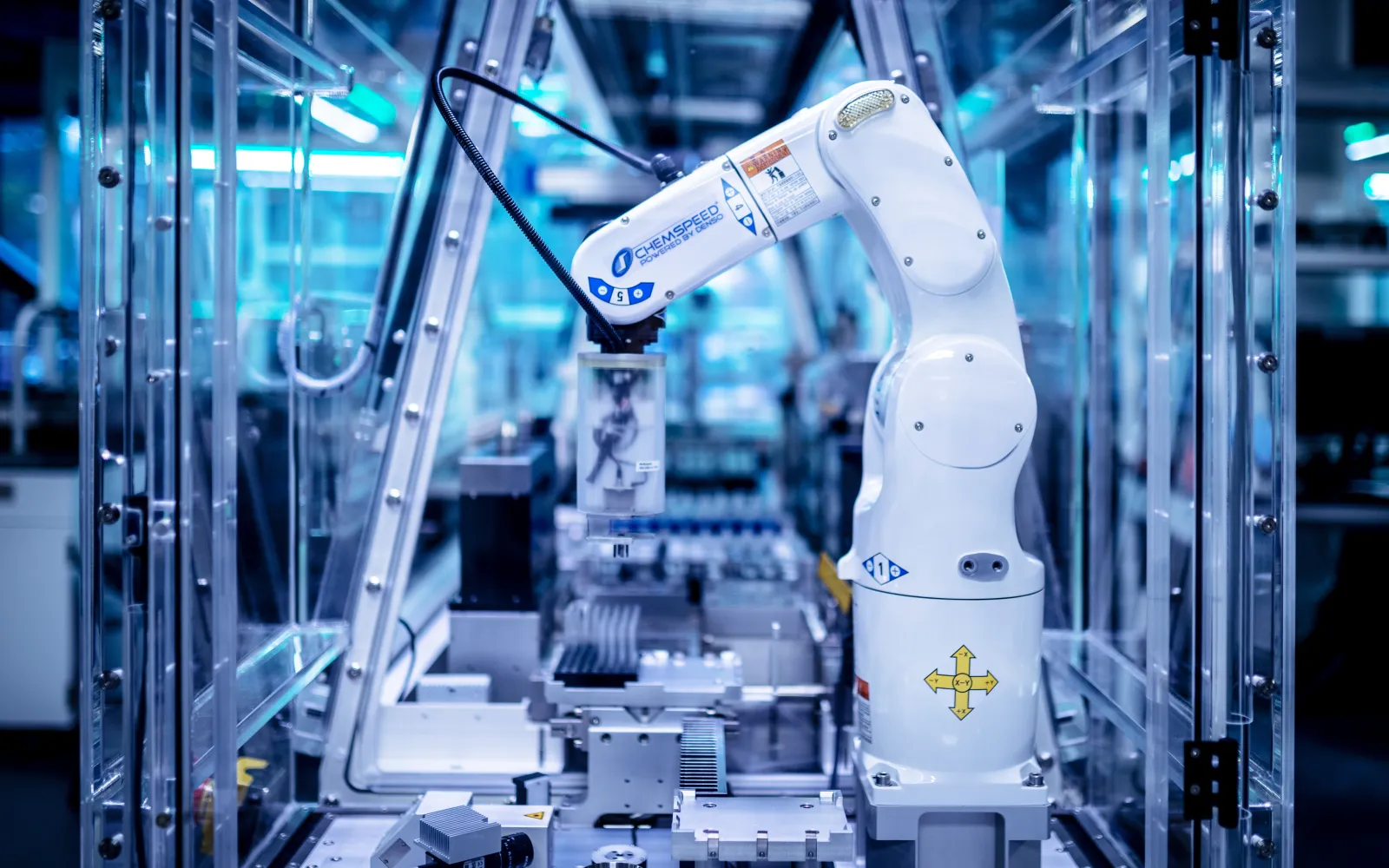
- Zymergen, which helped to accelerate and support critical COVID-19 testing initiatives by volunteering 30 of its world-class science automation team members, robots, equipment and consumables to a number of regional testing and therapeutic companies, including the Chan Zuckerberg Biohub, where key Zymergen staff delivered, set and activated specialized equipment on site in less than 48 hours. The company also helped drive and scale COVID-19 testing for sister DCVC company Curative, and continues to reach out to other entities to provide similar assistance.
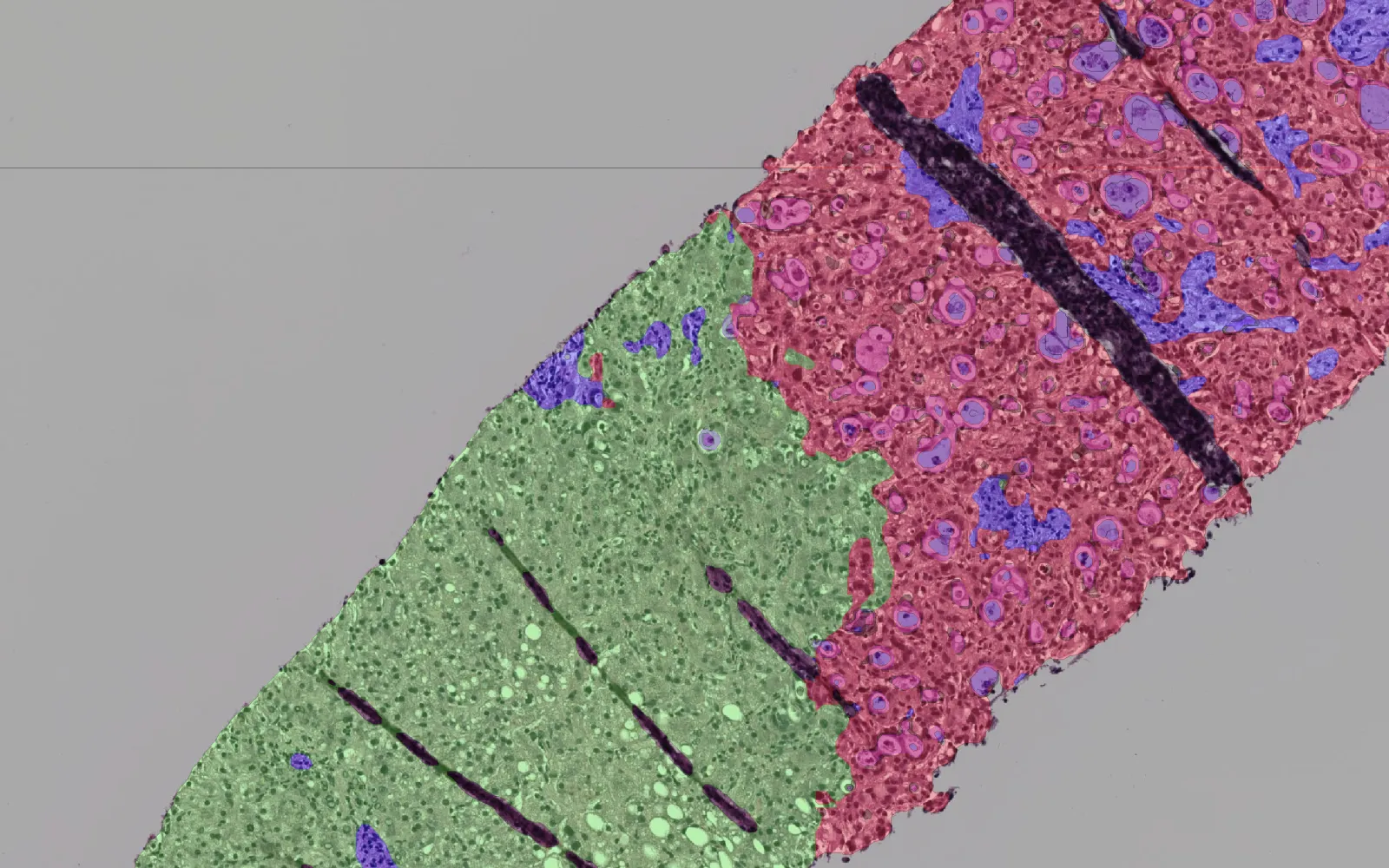
- Recursion has partnered with a local Biosafety Level 3 facility to screen thousands of FDA-approved and Generally Recognized as Safe compounds in an unbiased, morphological, AI-enabled search for unexpected signals of efficacy against SARS-CoV‑2 in primary human cells. The company will share any useful discoveries made from conducting experiments with the live SARS-CoV‑2 virus with colleagues at NIH/CDC/FDA as well as the biotech community, and will not seek any IP or profit on discoveries made as part of the project.
For other DCVC companies, this pandemic is a timely reminder of how the work they are already contributing to help increase efficiency and produce better outcomes in patient care and therapeutic discovery can benefit our society in a crisis such as this:
- Caption Health delivers FDA-authorized AI guided cardiac ultrasound imaging software that can be used by nurses and other frontline healthcare providers on portable ultrasound equipment. While COVID-19 causes primarily pulmonary complications, it can have a direct effect on the heart – many patients in China and Italy died of acute cardiac complications as a result of contracting COVID-19. Additionally, patients with underlying cardiovascular disease are at higher risk of contracting COVID-19 and have a worse prognosis. Caption AI can enable rapid assessment of cardiac function, allow institutions to reduce the risk of COVID-19 exposure for personnel, and stretch limited resources by enabling more healthcare workers to perform echocardiograms for patients in isolation. It is currently being deployed to select partners.
- Medical Informatics Corp has an FDA-cleared, software-based monitoring and analytics platform for ICUs which can help hospitals effectively increase bed capacity without new staff, consumables, beds or equipment. It can be stood up in any of the new pop-up facilities being built to provide extra capacity in this pandemic and means that providers – even those in isolation – can help monitor patients from afar to help provide triage support to care teams on the ground.
- Strateos has fully automated drug discovery and synthetic biology research and has partnerships with major pharmaceutical companies like Eli Lilly. Using advanced robotics to synthesize, evaluate, test drugs from scratch against complex wet lab processes, it is essential for accelerating the discovery and testing of drugs to treat COVID-19.
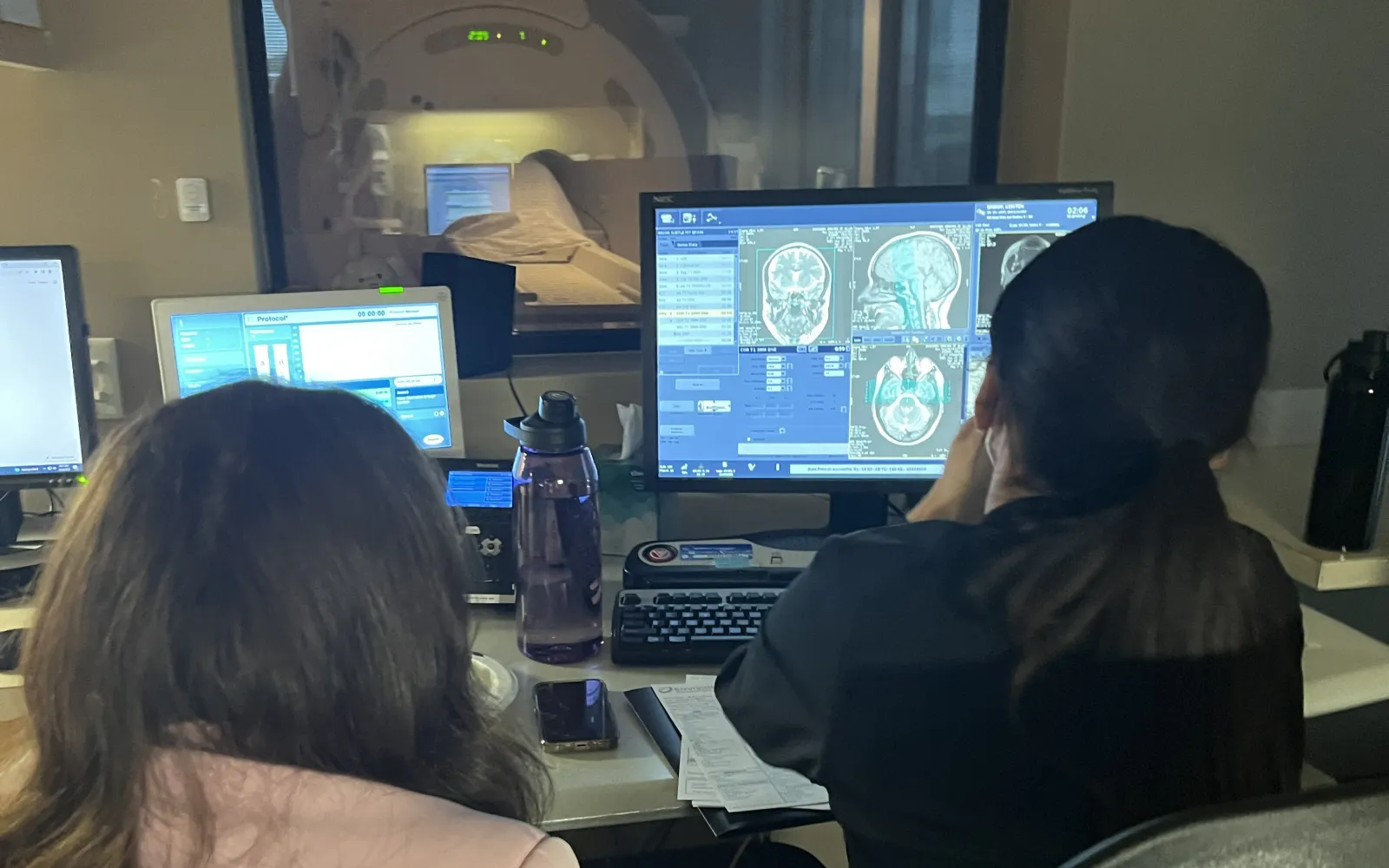
- Subtle Medical is a healthcare technology company with 2 FDA-cleared AI-powered software solutions for faster PET-CT and MRI currently in clinical usage at hospitals and imaging centers. While some elective scans can be postponed, many cancer patients in need of PET scans cannot afford to wait. Subtle Medical’s SubtlePET™ software can potentially help support the influx of imaging volume during the current COVID-19 crisis by making PET scans up to 75% faster, therefore making room for additional CT scans to be performed on the PET-CT scanner. Subtle is also currently assessing the need to accelerate the development of a lower-dose CT product currently in research phase for hospitals that prefer a safer CT option to measure COVID-19 progression.
The coming days and weeks will be difficult for all of us as we confront the full impact of COVID-19. At DCVC, we are encouraged by the tremendous effort of the medical and scientific communities in building a comprehensive response to this pandemic, from spinning up new field ICU facilities to speedily identifying new treatments. For all our portfolio companies taking an active role in the response to COVID-19, we are proud to support you and thank your teams for the immense effort you are making during this stressful and challenging time. And for our broader community, we hope you and your families continue to stay safe and healthy.
We’ll continue to look for more opportunities to support Deep Tech companies offering concrete solutions that will not only have an impact in the face of this pandemic, but will change our response to things like it in the future. For more information about the global response to the COVID-19 pandemic, please visit: https://www.who.int/emergencies/diseases/novel-coronavirus-2019