Kanvas Biosciences
Mapping the microbiome to improve human health
The health of the tissues we think of as ours — the 30 – 40 trillion cells that carry human DNA — depends on the health of the other 40 trillion or so microbial cells that live in our guts, on our skin, in our lungs, and in other tissue. Disorders of the microbiome, or dysbioses, are implicated not just in conditions like inflammatory bowel disease and celiac disease but also in allergies, obesity, cardiovascular problems, and even the way cancer patients respond to immunotherapy. So optimizing a malfunctioning microbiome with a mix of living microbes could be a promising way to treat or prevent a range of human health problems.
But up to now, drug developers creating these live biotherapeutic products, or LBPs, have faced two challenges — roadblocks that led to years of disappointment in the field of microbiome science. First, there hasn’t been an efficient, FDA-approved way to manufacture and deliver complex LBPs with the right communities of microbes to reconstitute a healthy microbiome. As a crude alternative, doctors can fall back on fecal microbiota transplant or FMT from a healthy donor. And sometimes that works: fecal transplants have been shown to help eliminate pathogenic bacterial strains like Clostridioides difficile. However, FMTs are by their nature inconsistent, and cannot be scaled or optimized.
Second, and relatedly, we’ve lacked methods for documenting exactly where LBPs end up in the body and whether they’re interacting with host cells in the intended fashion. In traditional drug development, you need to understand how a substance is absorbed, distributed, metabolized, and eliminated, or you can’t determine how it should be dosed, let alone whether it’s safe and effective. This is just as true for LBPs as it is for traditional small-molecule drugs.
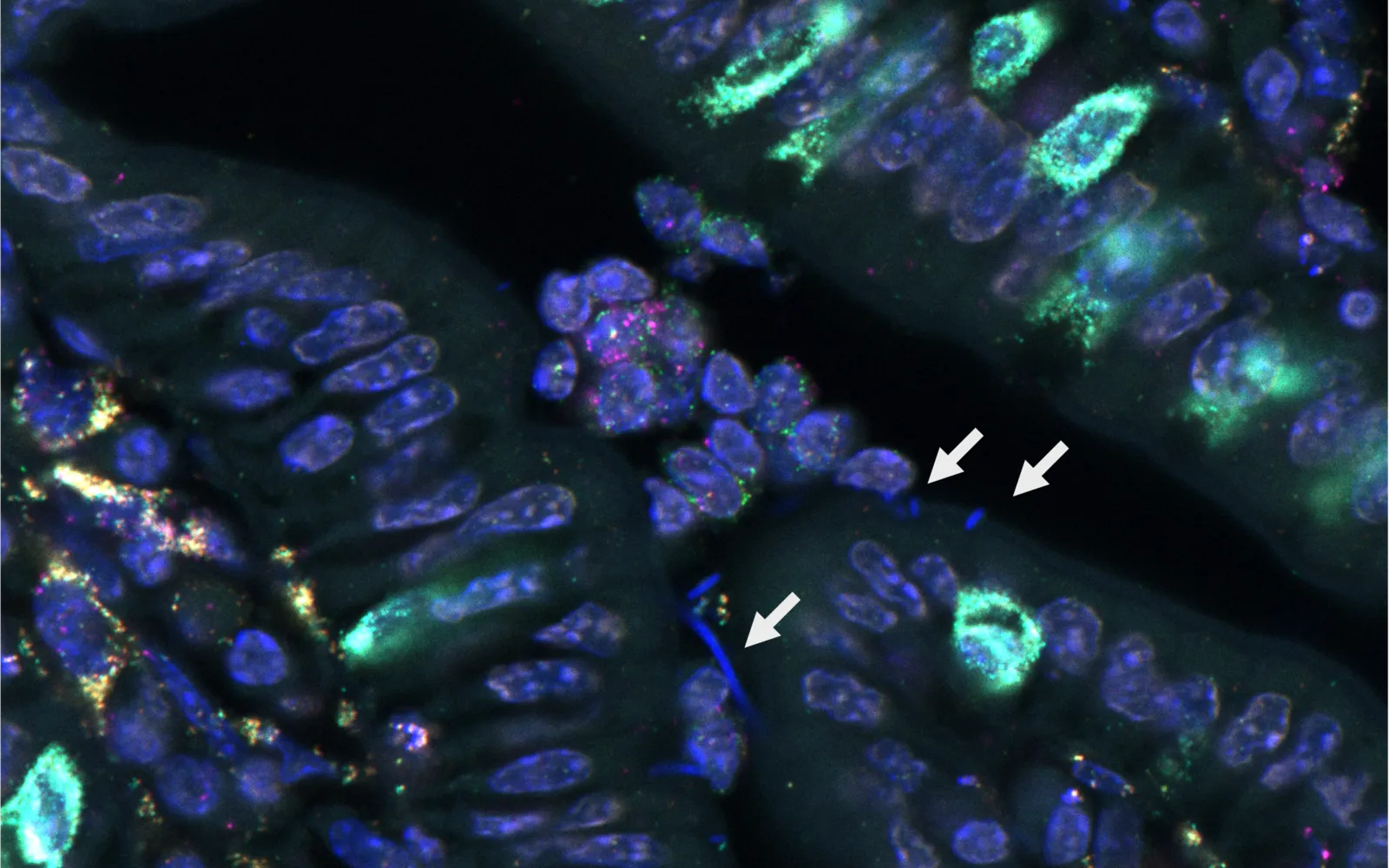
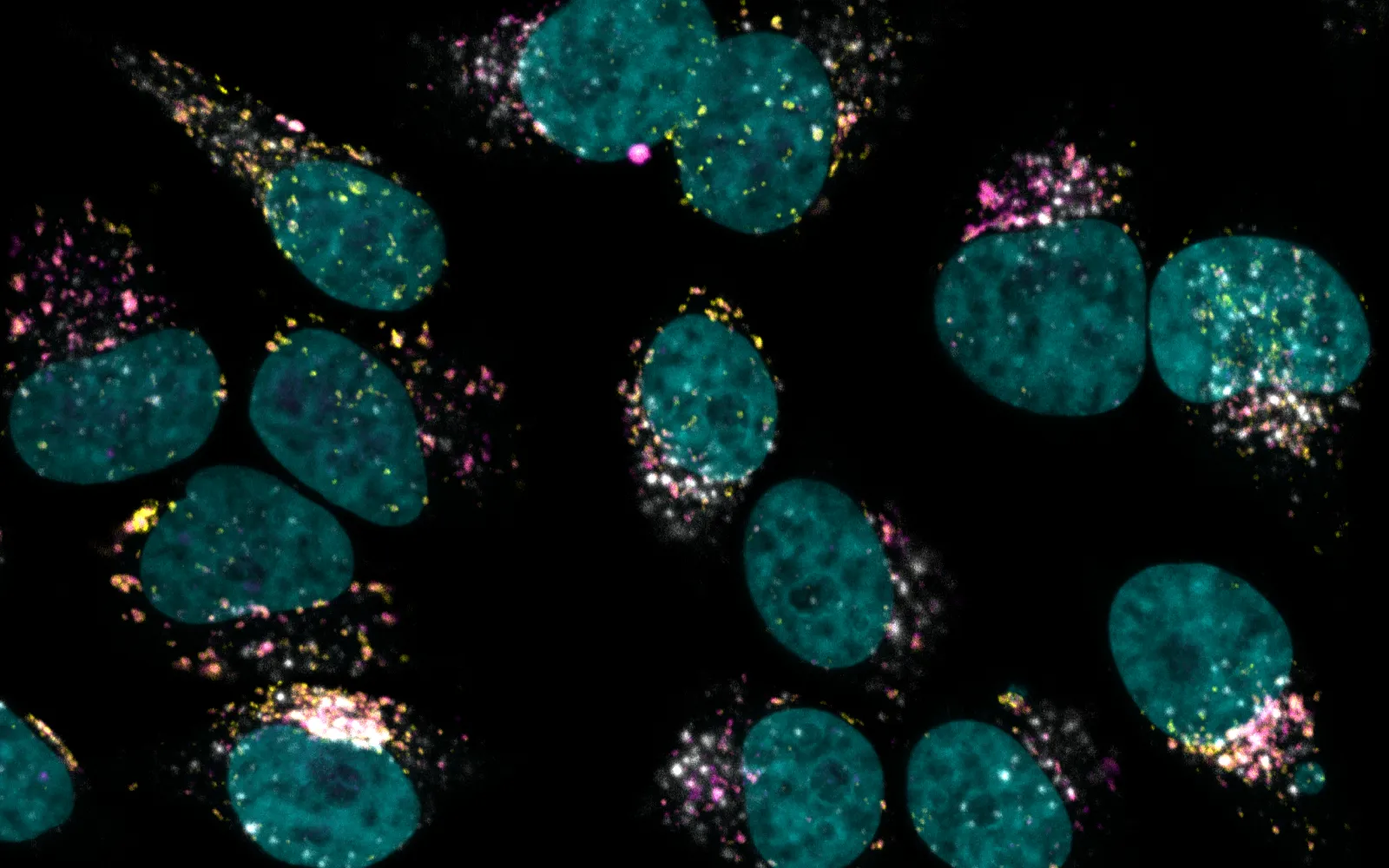
It was to help solve the second problem that DCVC led a $12 million funding round for Kanvas Biosciences in June 2023. The company’s co-founders invented an ingenious spectral barcoding system that, when combined with machine learning, can identify and pinpoint the RNA transcripts being expressed by both microbial cells and host cells. That means the Princeton, NJ-based company can directly visualize LBP engraftment and activity — key parameters required to design safe and effective therapies.
Now that it can help elucidate how LBPs act on our cells, Kanvas is also leapfrogging toward a solution for the first problem: how to make LBPs that contain complex consortia of beneficial bacteria. The company just announced that it acquired the manufacturing technology, key intellectual property, and two active clinical programs from Federation Bio, a South San Francisco-based developer of microbiome therapeutics with one of the most advanced facilities for assembling groups of microbes with desirable functions. The two teams have already worked together for years on microbiome projects requiring both of their platform technologies. See Fierce Biotech coverage of the news here.
Kanvas co-founder and CEO Matt Cheng explains the significance of the acquisition this way: “Kanvas invented a novel spatial biology platform that can profile host-microbe interactions. The logical question was, how do you generate value from that? Federation Bio was the world leader in biomanufacturing complex, high-diversity live biotherapeutic products. By combining a breakthrough discovery platform with first-in-category manufacturing, I think we’ve created a company that’s uniquely positioned to realize the promise of microbiome science.”
Recreating Complex Microbiomes
We agree, and that’s why DCVC worked with Kanvas to acquire specific assets from Federation Bio.
Federation Bio was founded to create therapies containing controlled mixtures of gut bacteria. Through metagenomic sequencing of multiple fecal samples, the company identified strains that contribute to a healthy microbiome. Then it put together smaller subsets of those strains that still have the metabolic properties fundamental to human health — such as the gene-protein pathways needed to break down dietary fiber and make butyrate, a short-chain fatty acid that fuels the cells lining the colon.
Early on, the company won the FDA’s blessing to co-culture many of these strains together in a single fermenter. “That opened up this field to us, to be able to recreate complete synthetic microbiomes that have over 100 different members,” says Lee Swem, Kanvas’ chief development officer. “It means we can design therapies that have multiple, complementary mechanisms of action to enable better engraftment and disease control across more patients.”
In an ongoing program now being overseen by Kanvas, Swem and his team sought out donors who responded positively to cancer immunotherapy and created cell banks that embody the diversity of their microbiomes. The company now has the opportunity to create optimized microbial communities that will enhance the immunotherapy response of all cancer patients. A second clinical program at Kanvas is designed to test synthetic communities of microbes that could treat inflammatory bowel disease, a painful and debilitating condition that affects more than 2 million Americans.
The eventual goal is to bypass FMTs altogether and administer biosynthetic communities of microbes to patients in a simple oral formulation. “We’re doing everything under the sun to make it work,” Swem says. “But we’re going to get there in a step-wise fashion, by carefully formulating and delivering these consortia in a manner that can positively impact the most patients, and by using Kanvas’s spatial biology capabilities to measure LBP engraftment, safety, and efficacy.”
Paddling in the Same Canoe
In a single transaction, Kanvas has transformed itself from a New Jersey startup with a promising imaging platform into a bicoastal company with a cell banking laboratory and two clinical development programs. “We knew we had the opportunity to apply our platform to the discovery and development of our own LBPs, but this will be a lot faster,” Cheng says.
The immuno-oncology program will be the first child born from the union of the Kanvas and Federation Bio platform technologies. “We have the opportunity to really leverage our technology platform to inform the design, engraftment, safety, and efficacy” of the company’s newly acquired experimental treatments, says Cheng. “Imagine two people paddling in the same direction but in different canoes, and you put them in the same canoe. You can go twice as fast in the same direction.”
Jason Pontin is a General Partner at DCVC and a cofounder of DCVC Bio portfolio company Totus Medicines.